| |
|
|
| |
|
|
| |
Affinity
Chromatography based on Glass Fiber Membrane |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
- Introduction
Affinity membranes containing trypsin and papain immobilized as
affinity ligands were prepared from glass fiber filters. The filters
were first treated with a concentrated solution of H2SO4
+ H2O2 (piranha
solution), and then modified by silanization to introduce epoxyl
or amino functional groups, which were further modified to carboxyl
or aniline moieties, respectively. Onto the modified membranes,
bovine serum albumin, trypsin and papain were immobilized as ligands.
Three immobilization methods based on glutaraldehyde, carbodiimide
and diazotization were used and compared to conclude that the
one based on glutaraldehyde, which can form a crosslinking structure
among the membrane, was the most efficient.
|
|
| |
|
|
| |
|
|
| |
- XPS Characterization
of Glass Surface Modification
Besides the introduction of suitable functional groups, the silanization
also helped to generate a uniform layer over the glass surface
by capping the high potential sites, which stimulated irreversible
protein adsorption when the membranes were used for filtration
[31]. Figures 1 (b) and (c), show that after silanization, no
metallic ions are present on the surface of the glass, which means
that the ions present in Figure 1 (a) were all capped, and that
the surface of glass was covered by a silane compound.
|
|
| |
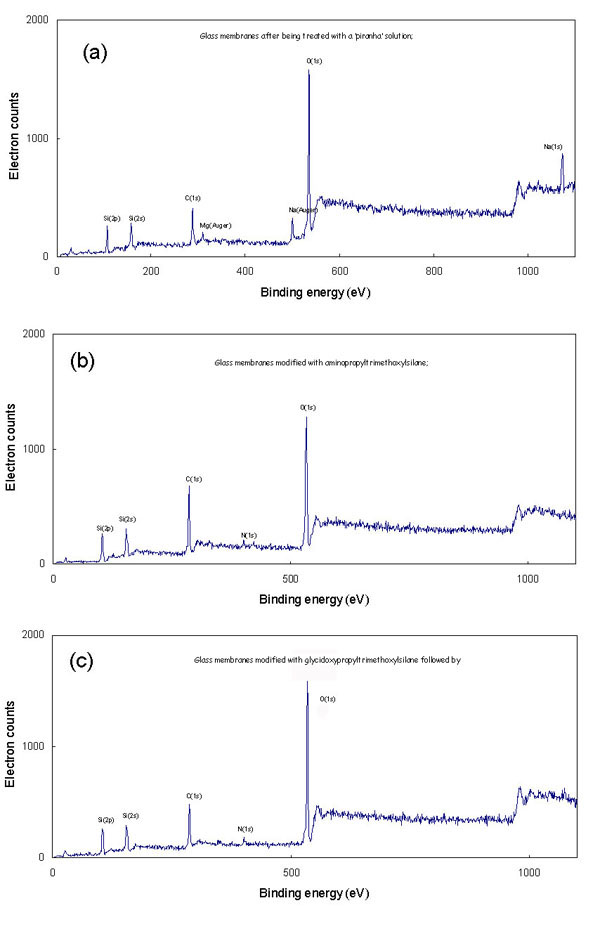
XPS Spectra of the glass fiber
membranes
|
|
| |
|
|
| |
|
|
| |
- Membrane Preparation
The glass filter was first treated with piranha solution, and
then modified with gamma-glycidoxypropyltriethoxysilaneg-glycidoxypropyltriethoxysilane
to introduce amino groups.
|
|
| |
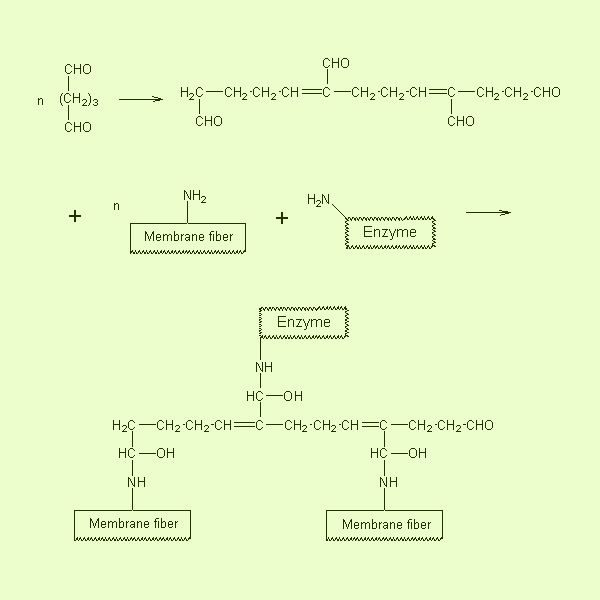
Crosslinking of Glass Fibers with Glutaraldehyde
|
|
| |
|
|
| |
|
|
| |
- Proof of Crosslinking
The activities
of the immobilized trypsin, prepared by the three methods (glutaraldehyde,
diazotization and carbodiimide), are compared in the following
figure (dynamic stability). One can conclude that the activity
of the immobilized trypsin prepared via the carbodiimide method
is comparable to that prepared via diazotization and that both
decrease with the number of determinations (washings). These occurred
because, in contrast to the glutaraldehyde method, no crosslinking
which stabilizes the structure took place during immobilization.
|
|
| |
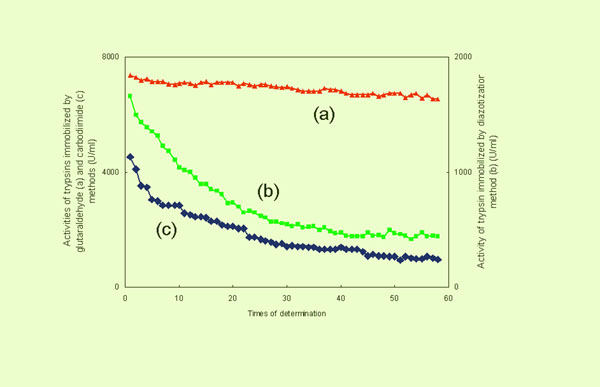
Dynamic Stability of Immobilized Trypsins Prepared by Three Methods
|
|
| |
|
|
| |
|
|
| |
- Biocompatibility
of Prepared Membrane
The stabilities of the affinity
membranes are controlled by two important factors: (i) the resistance
to the washing by the buffer (the dynamic stability), and (ii)
the durability to denaturation (biocompatibility). The biocompatibility
of the glass membrane (static stability) was investigated by determining
the rate of denaturation of the immobilized trypsin. The figure
shows that all three kinds of immobilized trypsin were very stable
at room temperature, indicating a high biocompatibility of the
glass membrane.
|
|
| |
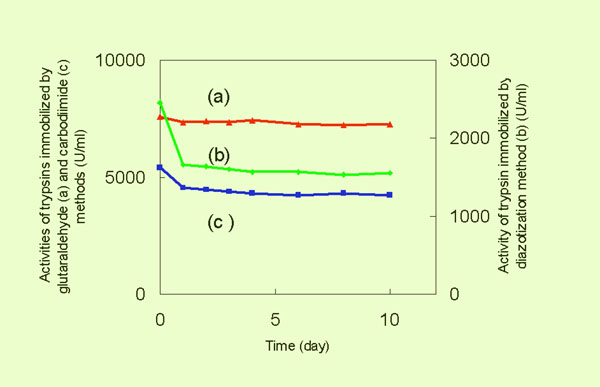
Static Stability of Immobilized Trypsin on Glass Membrane
|
|
| |
|
|
| |
|
|
| |
- Separation of Papain
Inhibitor from Potato Tuber
|
|
| |
|
|
| |
|
|
| |
|
|
| |
- Comparison of the
Crosslinking Methods
Besides the
glutaraldehyde method, the glass membranes were also crosslinked
with the imine or ethylene bifunctional silane, and the effects
were examined by the EMS
|
|
| |
|
|
| |
|
|
| |
|
|
| |
- Separation of Fibronectin
from Human Plasma using Gelatin followed by Heparin Affinity Membranes
|
|
| |
 |
|
| |
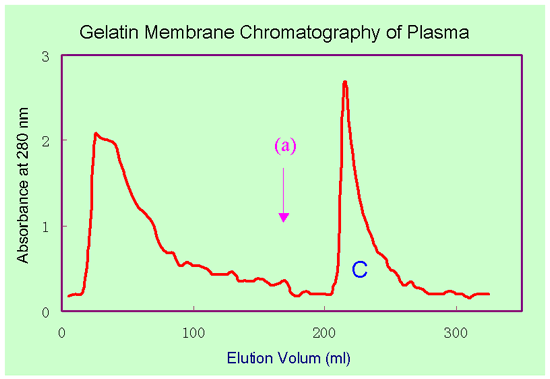
A 40
ml reconstituted human plasma was applied to a gelatin affinity
membrane cartridge (20 sheets of membranes, 47 mmID and 8 mm thickness).
The bound proteins were eluted with 3 M Urea in buffer.
|
|
| |
|
|
| |
The
product from the above procedure, after dialysis, was applied to a
heparin affinity membrane cartridge (20 sheets of membranes, 47 mmID
and 8 mm thickness). The
bound proteins were eluted respectively with 250 mM NaCl and 500 mM
NaCl in the same buffer. |
|
| |
| |
A
HSA
B Human plasma
C Product from gelatin chromatography
D E F Fractions from Heparin chromatography
G Fibronectin (Sigma) |
|
|
| |
|
|
| |
|
|
| |
|
|