| |
|
|
| |
|
|
| |
Affinity
Chromatography based on Cellulose Membrane |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
- Membrane Preparation
Mercerized macroporous cellulose membranes with large pore sizes
(0.5~1 micron) and high porosity (about 55%) were prepared from
filter paper by Mercerization (caustic treatment) followed by
chemical crosslinking.
|
|
| |
|
|
| |
|
|
| |
- Effect of
Mercerization
During Mercerization,
swelling occurred, and some crystalline I domains were converted
into crystalline II and amorphous domains. The following shows
that during Mercerization the diameter of the cellulose fiber
increased and that its smoothness was reduced. The figure also
shows that the thickness of a Mercerized membrane expanded. However,
when the membranes were packed into a cartridge under pressure,
the thickness of the Mercerized membrane became even somewhat
smaller than that of the original non-Mercerized membrane. This
indicates that Mercerization increased the softness of the fibers.
|
|
| |
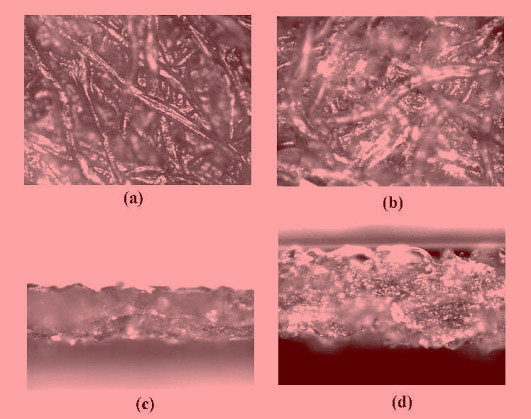
Micrographs of crosslinked cellulose membranes
(a) (c) before Mercerization, (b) (d) after Mercerization |
|
| |
|
|
| |
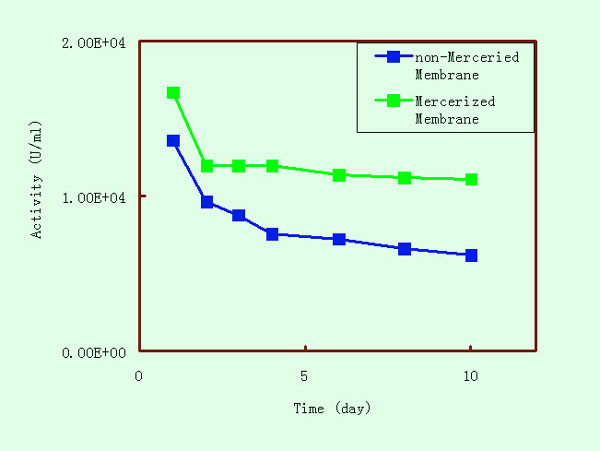
Stabilities of Immobilized trypsins on Mercerized and non-Mercerized
Membranes |
|
| |
|
|
| |
|
|
| |
- Effect of
Crosslinking
The woven
fibers of cellulose became by this means linked each other and
fixed firmly. Compared with its precursor, the product possesses
a tighter structure with less free fibers.
|
|
| |
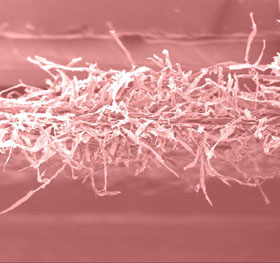
(A) |
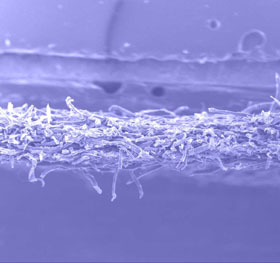
(B) |
|
|
| |
The
SEM Photos of Cellulose Membrane (B) and Its Precursor (A). |
|
| |
|
|
| |
|
|
| |
- Biocompatibility
of Prepared Membrane
The stability of the immobilized
trypsin, determined as indicated in the experimental part, was
investigated and the results are presented in the following figure.
The experiments were performed in severe conditions for the immobilized
enzyme, namely, the room temperature. The immobilized trypsins,
prepared using the three activation methods, were very stable
during at least one week, indicating that the Mercerized crosslinked
cellulose membrane provided a good matrix for affinity chromatography.
The immobilized trypsin prepared by the epoxy method possessed
the highest stability, most likely because it also exhibited the
lowest activity and hence was less subjected to change.
|
|
| |
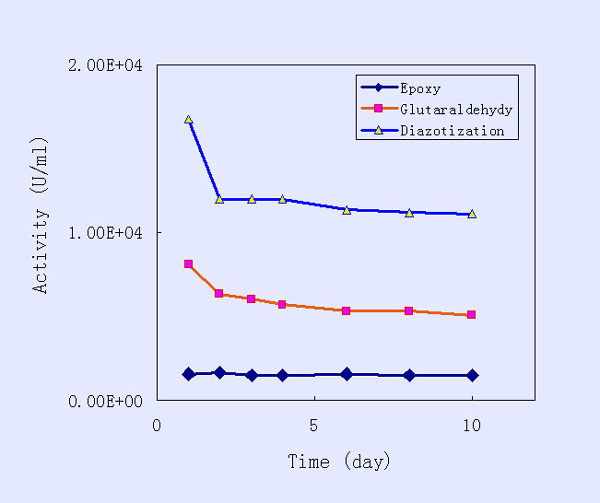
Stability of Immobilized Trypsin on Cellulose Membrane
|
|
| |
|
|
| |
|
|
| |
- Immobilization of
Concanavalin A
|
|
| |
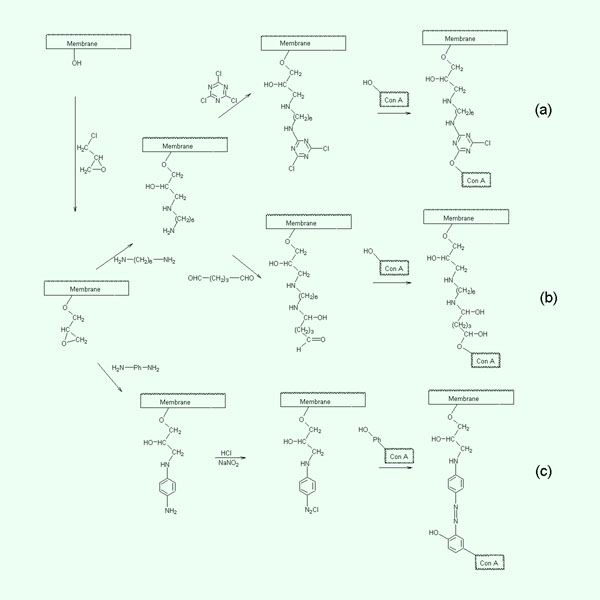
Immobilization of Concanavalin A by (a) Triazine, (b) Glutaraldehyde
and (c) Diazotization Methods |
|
| |
|
|
| |
|
|
| |
|
|
| |

Immobilization of Enzyme by (a) Epoxy, (b) Glutaraldehyde and (c)
Diazotization methods |
|
| |
|
|
| |
|
|
| |
- Immobilization
of Maltose
|
|
| |
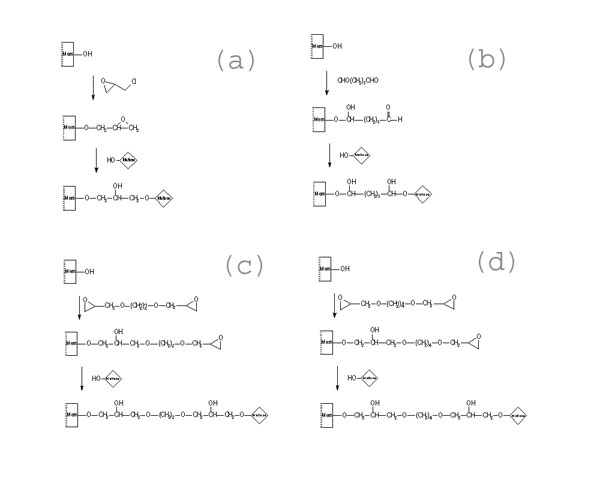
Immobilization of Maltose via Spacers of
(a) 5-atom, (b) 7-atom,
(c) 10-atom and (d) 12-atom |
|
| |
|
|
| |
|
|
| |
- Purification of
Ovamucoide from Egg White
Trypsin immobilized affinity
membrane was used for the separation of trypsin inhibitor (ovomucoide)
from the egg white, and a product with high purity was obtained.
This procedure is advantageous because the membranes possess fairly
large pore sizes and porosities, and only a simple pretreatment
(centrifugation) was necessary to remove the insoluble from the
solution of egg white.
|
|
| |
|
|
| |
|
|
| |
|
|
| |
- Separation
of Papain Inhibitor from Potato Tuber
A wide elution peak of the papain inhibitors is present
in following chromatogram, from which three fractions at 70, 80
and 90 min were selected to examine their purity by electrophoresis
(Sample B, C and D in electrophoretic result). As shown in electrophoresis,
the elution peak involves at least three types of papain inhibitors.
Because the amount of impurities became smaller in the later stages
of elution, the final purity of the papain inhibitors was enhanced.
|
|
| |
|
|
| |
|
|
| |
|
|
| |
- Separation of Peroxidase
from Horseradish
Compared to column chromatography, the method using concanavalin
A affinity membranes has three main advantages: (1) A single chromatographic
procedure is involved; (2) The affinity membranes employed are
more stable; and (3) A higher loss of enzyme activity is expected
to occur during the ion exchange chromatography than during dialysis.
|
|
| |
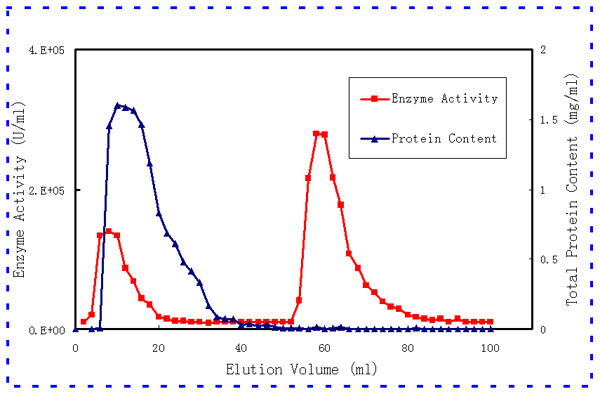
Separation of Peroxidase from Horseradish
by Concanavalin A Affinity Membrane |
|
| |
|
|
| |
|
|
| |
- Purification of
Concanavalin A
As an application,
the commercially available crude con A was purified. Con A is
a common affinity ligand that is mostly used to separate saccharides
containing molecules, such as glycoproteins, some enzymes, IgGs,
interferons and some protein hormones. In this paper, the crude
con A was purified by using a membrane activated with 1,4-butanediol
diglycidyl ether, upon which maltose was immobilized. The chromatogram
of con A is presented in the following figure, where the shape
of con A peak indicates a good separation efficiency. The quality
of the purification achieved was determined by electrophoresis.
The results show that the purified con A exhibits a single band.
|
|
| |
|
|
| |
|
|
| |
|
|
| |
- Purification of
Phosphatase
Triazine dyes
can serve as an analogue for nucleotide so that a protein will
bind them at nucleotide binding sites. 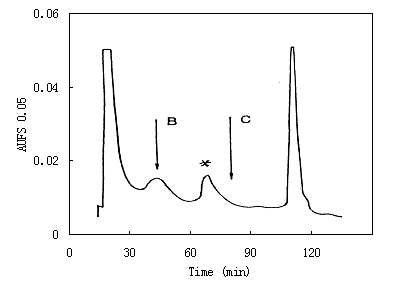 They
have been used successfully in the purification of many enzymes,
especially kinases and dehydrogenases. The phosphatase can be
purified on dye immobilized medium. In this work, commercial alkaline
phosphatase from calf intestine was purified on the affinity membrane
cartridge containing 80 sheets red cellulose membranes. The phosphatase
was eluted by 1 M NaCl, and an unknown component with stronger
affinity to the ligand was eluted by 60% glycol.The recovery of
alkaline phosphatase activity was 60% and a 40-fold purification
was achieved. They
have been used successfully in the purification of many enzymes,
especially kinases and dehydrogenases. The phosphatase can be
purified on dye immobilized medium. In this work, commercial alkaline
phosphatase from calf intestine was purified on the affinity membrane
cartridge containing 80 sheets red cellulose membranes. The phosphatase
was eluted by 1 M NaCl, and an unknown component with stronger
affinity to the ligand was eluted by 60% glycol.The recovery of
alkaline phosphatase activity was 60% and a 40-fold purification
was achieved.
|
|
| |
|
|
| |
|
|
| |
- Selfcleaning of
Trypsin Affinity Membrane
|
|
| |
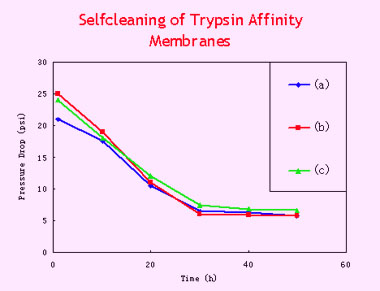 Since
the immobilized trypsin exhibits a high activity and stability, the
affinity membranes can self-clean themselves, after their permeability
was decreased by fouling. In this paper the self-cleaning could be
easily carried out by incubating the cartridge in a suitable buffer,
which, activating the immobilized enzyme, could hydrolyze the proteins
retained inside the membrane. The permeability of the cartridge could
be thus restored to its original level. Since
the immobilized trypsin exhibits a high activity and stability, the
affinity membranes can self-clean themselves, after their permeability
was decreased by fouling. In this paper the self-cleaning could be
easily carried out by incubating the cartridge in a suitable buffer,
which, activating the immobilized enzyme, could hydrolyze the proteins
retained inside the membrane. The permeability of the cartridge could
be thus restored to its original level. |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|